Extreme temperatures, high/low light intensity, draught, flooding, salinity are, to name a few, stressors that plants will be repeatedly subjected to over the course of their life-time. As a result, due to their "sedentary life-style", plants must adapt quickly to their ever-changing environment if they want to survive. Understanding how plants sense, respond and adapt to these stressors is of primordial importance since it has recently been estimated that the temperature increase due to global warming this century will likely exceed previous predictions,[1] hence increasing environmental stress on plants not only by their intensity but also by their frequency. As plant domestication occurred under more favourable conditions than during the early evolution of land plants, crops have been selected on their productivity rather than on their resistance to abiotic stress and with the world population predicted to reach 9.7 billion by 2050, plant scientists face one of the biggest challenge of the future: increasing crop production on less area and with dwindling resources of water.[2]
How do plants respond to draught stress?
It is usually assumed that plant response to stress happens in three different phases.[3]
- An alarm phase, when plants detect a change in their environment and activate different mechanisms to be able to cope with the change.
- A resistance phase, where plants adjust their structure and function in order to withstand the stress and repair the damaged caused.
- If the stressor stops or lessens then the plant may recover and reach a new optimal physiological status. However, if the stress continues or is too intense, then the plant dies.
In the specific case of draught stress, plants are known to reduce photosynthesis by decreasing their leaf area as well as their photosynthesis rate, mainly by inhibiting the CO2 mechanism and by restricting the diffusion of CO2 into the leaf via stomatal closure. As a result of this and because of the low concentration of intracellular CO2, ongoing photosynthetic light reactions may cause the building-up of reduced photosynthetic electron transport components, which can react with molecular oxygen to form highly damaging reactive oxygen species (ROS).[4]
As a result, plants cope with stress by modulating key physiological processes that result in the modification of molecular and cellular processes. This plasticity is mediated by phytohormones such as abscisic acid (ABA), ethylene, cytokinin (CK), gibberellic acid (GA) and auxin as they play important roles in every step of plant development and hence will enable a plant to respond to abiotic stress. For instance, under drought stress, ABA is involved in stomatal closure whereas cytokinins are known to delay leaf senescence and death.[4]
Agrisera antibodies - Working towards a better understanding of environmental stresses in plant research
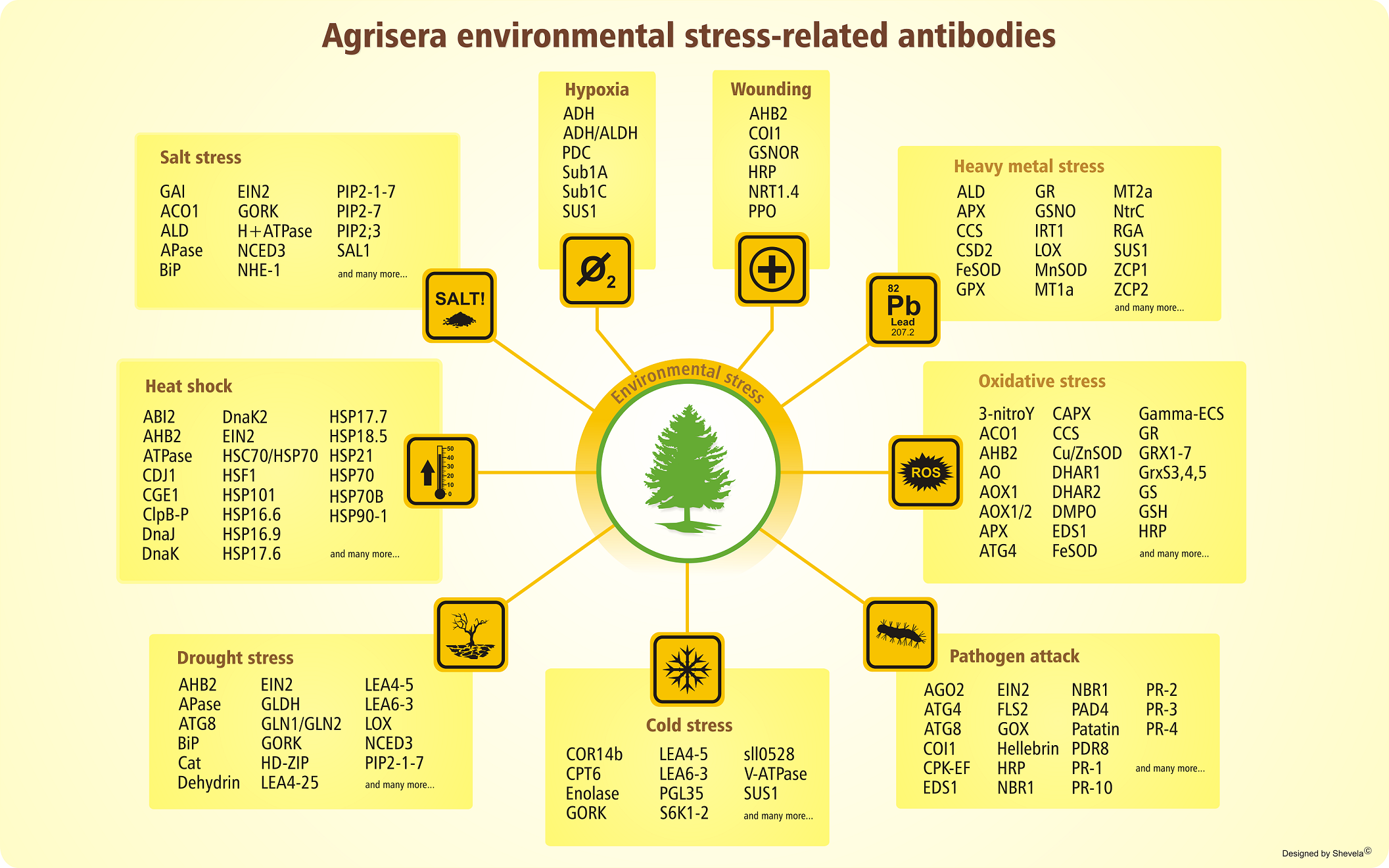
Development of crops and plants highly resilient to environmental stresses without compromising on yield is one of the many challenges that plant scientists are currently facing. To facilitate research in this area, Agrisera has developed an extensive range of plant antibodies. This includes environmental stress antibodies as well as phytohormone antibodies.
References:
1. Greater future global warming inferred from Earth’s recent energy budget, Brown PT et al, Nature 2017, 552, 45–50
2. Plant abiotic stress challenges from the changing environment; Pereira A, Front Plant Sci., 2016; 7: 1123.
3. Stress Memory and the Inevitable Effects of Drought: a physiological perspective; Fleta-Soriano E et al, Front Plant Sci, 2016,7, 143
4. Plant adaptation to drought stress, Basu S et al, Version 1. F100Res. 2016; 5: 1554.
Written by Magalie Dale
If you like my post why not connect to me on LinkedIn.